Even small amounts of dissolved nonpolar gases (CO2, CH4, N2) can significantly decrease the dielectric permittivity of the fluid. In the present communication the method for account of this effect in mixed fluids on the complexes formation reactions is proposed. On the base of the method treatment of evolution of model Pb(II)-CO2-NaCl-H2S-H2O fluid was carried out. It is shown that the diminishing in the dielectric constant because of CO2 presence in the fluid dramatically changes all the chemical behaviour of aqueous components and may result in unconventional mechanism of galena's formation.
The water dissolution of nonpolar gases (CO2, CH4) gives rise to the reduction of dielectric constant em of the medium. To compute em it could be set that additive is the e1/3 value. So for H2O-CO2 mixture
em = Fw×ew1/3 + FCO2×eCO21/3
where Fw and FCO2 - volume fractions of H2O and CO2 with relative permittivities ew and eCO2. Decreasing in em will accent the association reactions equilibrium to the products. For the detailed calculation of this effect it is convenient to use HKF state equation (Tanger and Helgeson, 1988): change in em acts on the solvation part of Gibbs free energy of aqueous species DGs = w×(1/em - 1), where w = h×Q2/r refers to the absolute Born parameter of the species with charge Q and effective electrostatic radius r [Å], and h = 1.660277×105cal×Å/mole. So to compute equilibrium in the mixed fluid it is necessary to modify standard chemical potential of every aqueous species such as
DGs = w×( 1/em - 1/eH2O ) (1)
This approach enables oneself not to confine within examination of isolated reactions, but study equilibria of the whole system.
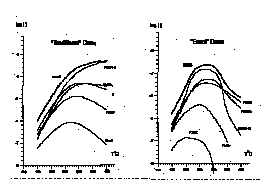
With the goal to estimate change in em impact on the chemical properties of model H2O-CO2-NaCl-H2S fluid, calculations of PbS (galena) solubility were executed under typical natural ore formation conditions. The initial concentration of CO2 was set to be 9.5 mole/kg that corresponds to the onset of exolution at 350oC. While the subsequent temperature lowering, CO2 concentration decreases slipping along the saturation curve. The thermodynamic data from SUPCRT92 (Johnson et al., 1992) were applied. Using the proposed method detailed composition of the fluid while lowering temperature was computed in two cases - without (so called traditional case) and with (exact case) chemical potentials' modification according to Tanger and Helgeson (1988). The result gives one's evidence that variation of em dramatically changes components' speciation of the solution. Also the behaviour of Pb in the solution does change too. For example at 400oC in the real fluid PbCl2o is superior to all other chloride complexes (right fig.), but not PbCl42- as it is assumed to expect in the traditional case (left fig.). This effect causes the PbS solubility curve to have particularly pronounced maximum at 275oC (right fig.). Such a "strange" behaviour of Pb could favour its overdepositing and concentrating while moving fluid is cooling down.
Thus the effect of dielectric permittivity change, which is associated with the exolution process, is considered to be a new powerful factor of ore formation and may reveal to the unconventional effects of element partitioning in mixed natural fluids.
Johnson, J.W., Oelkers, E.H. & Helgeson, H.C., Comp. Geosciences 18, 899-947 (1992).
Tanger, J.C. IV & Helgeson, H.C., Amer. J. Sci. 288, 19-98 (1988).
Fig. 1: Logarithms of galena's solubility mPbS [mole/kg] and equilibrium molal concentrations of Pb2+ and its chloride complexes for the model of fluid's evolution assumed in the study: a) stands for traditional and b) - for exact schemes of calculations.