The aim of this study has been to investigate the possible role of surface adsorption by iron sulphide surfaces in the scavenging and precipitation of gold from aqueous solutions. The adsorption of complex ions by sulphide surfaces has been little investigated even though such processes may be important for a wide range of conditions ranging from hydrothermal systems, both withing the earth's crust and on the ocean floor as well as to the earth surface environments. The dominant complexes responsible for gold transport by hydrothermal fluids in the crust are AuHS0 and Au(HS)2 (Renders and Seward, 1989a; Benning and Seward, 1996). We have therefore chosen to study the adsorption of hydrosulphidogold(I) complexes by pyrite over a range of pH and temperature in order to gain insight into the importance of sulphide surface adsorption processes in natural systems. Several previous such studies have been carried out by Renders and Seward (1989b) and Schoonen et al. (1992).
Pyrite was synthesised by mixing of an FeCl3.6H2O with an Na2S.9H2O solution such that the final concentrations were 0.034 molal and 0.067 molal respectively. The pH was adjusted to starting values of between 3.5 and 3.9 using hydrochloric acid. All solutions are prepared from deoxygenated water and to avoid oxidation, all the reactions and sample preparations took place in closed glass systems with nitrogen over pressure. During the mixing of the reactants, precipitation of amophous iron monosulphide occurs. The reaction vessel was immersed for 36h in a waterbath at 80°C, and then the product was separated from the solution by filtering and washing with 1M HCl to remove sulphide phases other than pyrite. The product was subsequently studied by XRD.
The point of zero charge of the pyrite was then determined by potentiometric titration and the BET surface area obtained by nitrogen adsorption.
Solutions containing gold(I) hydrosulphide complexes were prepared by the reaction of a sulphide solution with fine grained gold at near neutral pH. The dissolution of gold leads to concentrations of ~10ppm within 7 days. The solution must be stored in the dark to avoid photoreduction of the gold(I) hydrosulphide complexes.
The adsorption experiments were carried out in a
black painted, stirred reaction vessel containing a sulphide solution-pyrite suspension. Samples of the suspension were taken at varying intervals, filtered, digested in acid and analysed using atomic adsorption spectroscopy.
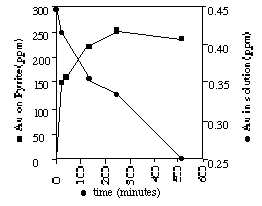
Figure 1 shows a graph of the first preliminary results. The experiment was at a pH of 7 and 25°C, where gold is present as Au(HS)2- species in solution. Within the first 18 minutes, gold up to 150ppm is accumulated by the pyrite surface. In the following 290 minutes, the gold content rises up to 250ppm were a plateau is reached. After 4 hours 24% of the dissolved gold is removed by adsorption, with a pyrite having a surface area of 3.4m2/g and a zero point of charge at a pH of ~2. Further changes in gold concentration within the solution may be due to the adsorption of gold by the pyrex glass of the reaction vessel.
Benning, L.G. & Seward, T.M., Geochim. Cosmochim. Acta 60, submitted (1996).
Renders, P.J. & Seward, T.M., Geochim. Cosmochim. Acta 53, 245-253 (1989a).
Renders, P.J. & Seward, T.M., Geochim. Cosmochim. Acta 53, 255-268 (1989b).
Schoonen, M.A.A., Fisher, N.S. & Wente, M., Geochim. Cosmochim. Acta 56, 1801-1814 (1992).
Fig. 1: Adsorption experiment: Gold concentration in solution and pyrite as a function of time after adding gold.