A dearth of basic thermodynamic data for ferric species does not permit quantitiative understanding of iron complex equilibria in high temperature aqueous solutions. The potentiometric technique is used in the present work in order to study Ox-Red equilibrium in the system containing ferric and ferrous iron.
The literature data on values of standard electrode potential E0 for the half cell reaction
Fe3+ + e- = Fe2+ (1)
obtained from the cell with liquid junction potential eliminated (Shumb et al., 1937; Bray and Hershey, 1934) and this one indefinite (Nikolaeva and Antipina, 1978) differ approximately on 0.025V from each other at 25°C. Therefore E was measured by two ways in this study. First run consisted of potentiometric titration of the HClO4 (0.0481-0.698M), Fe(ClO4)2, Fe(ClO4)3 (3*10-3M both) solutions by NaClO4(3M) with the same acidity and iron salts concentration in the cell with two Pt electrodes. The silver-silver
chloride reference electrode in saturated KCl and NH4Cl(3M) as liquid junction were used. E01 = 0.766±0.005V was obtained after subsequent procedures of accounting of ferric hydrolysis, extrapolation to zero acidity at constant ionic strengths and then to zero ionic strength in according with Debye-Huckel equation. Second run was performed in order to study temperature effect on E01. The cell
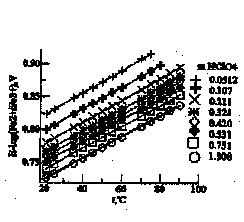
Pt,H2 ÍHClO4(m)ÍÍHClO4(m)Í (1)
ÍHClO4(m), Fe(ClO4)2(3*10-3m)ÍPt;
250C t=20.8-900C Fe(ClO4)3(3*10-3m)Ô
was used. HClO4 concentrations varied from 0.0512 to 1.308m. E01 =0.768 ±0.002V at 25°C and dE0/dT = 0.0008367V/K values were obtained after taking into account first hydrolysis constant of Fe3+ (Zotov et al., 1979), extrapolating to zero ionic strength using Debye-Huckel equation in III approach and correction for unisothermal potential of hydrogen electrode (Fig. 2). The data obtained in the present study check within experimental error with data of Whittemore and Langmuir (1972) but values of dE01/dT are different.
The standard thermodynamic functions of the reaction
Fe3+ + 0.5H2(g) = Fe2+ + H+ (2)
were calculated up to 350°C.
Bray, W. C. & Hershey, A. V., J. Amer. Chem. Soc. 56, 1889-1892 (1934).
Nikolaeva, N. M. & Antipina, V. A., Izv. Sib. Otd. AS USSR 6, 11-16 (1978).
Shumb, W. C. et al., J. Amer. Chem. Soc. 59, 2360-2365 (1937).
Whittemore, D. O. & Langmuir, D., J. Chem. Engineer. Data 17, 288-290 (1972).
Zotov, A. V. & Kotova, Z. Y., Geokhimiya 285-290 (1979).
Fig. 1: Measured values of E of the Cell (I) as functions of temperature.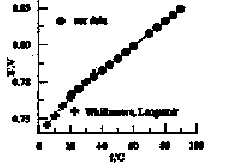
Fig. 2: Temperature dependence of E01.