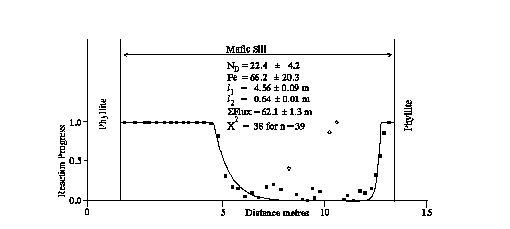
The kinetics of fluid-solid exchange in greenschist facies metamorphic rocks of the Dalradian of the S.W. Highlands, Scotland has been investigated by mapping progress of a carbonation reaction front and oxygen and Sr-isotope exchange. Mafic sills have been carbonated by infiltration of a CO2- bearing fluid from adjacent calc-phyllites. The asymmetric widths of the carbonated margins allows determination of flow direction and magnitude, and indicates that sill margins were buffered to nearly constant fluid composition by copious flow in the more permeable phyllites. Partially carbonated amphibole-epidote assemblages within the reaction fronts preserve evidence for sluggish reaction kinetics. An advective-diffusive transport model with linear reaction kinetics has been fitted to the reaction progress profile for a sill at Port Cill Maluaig, Knapdale (Fig. 1). This implies a time integrated fluid flux of 62.1 ± 1.3 m3/m2, a Damköhler Number of 22.4 ± 4.2 and a Peclet Number of 66.2 ± 20.3 (1<s errors). The Peclet Number and time integrated fluid flux imply that the flow event lasted between 0.02 and 20 Ma for plausible porosities in the range 10-3 to 10-6. The inferred rate constant for the reaction kinetics is two or more orders of magnitude slower than extrapolation of experimentally determined surface reaction rates (Walther and Wood, 1984). Either the kinetic dispersion arose from factors additional to surface reaction kinetics or fluid-solid reaction was controlled by a slower mechanism such as diffusion away from flow channels.
The Sr-isotope profile across the Port Cill Maluaig sill confirms the kinetic limitations on reaction rates and the extent of Sr-isotopic re-equilibration with the infiltrating fluid is related to the proportion of the original amphibole and epidote assemblage which has broken down to the products calcite, chlorite and quartz. The Rb-Sr isotopic systematics of the product minerals and plagioclase, recalculated at the time of metamorphism (520 Ma) allow the Sr-isotope profile of the infiltrating fluid to mapped and its Sr content recovered from mass balance given the time-integrated fluid flux derived from the reaction progress calculation.
Walther, J.V. & Wood, B.J., Contrib. Mineral. Petrol. 88, 246-259 (1984).
Fig. 1: Reaction progress calculated as stoichometric calcite/(calcite+amphibole) across sill at Port Cill Maluaig with best fit advection-diffusion with linear kinetic fluid-solid exchange infiltration model. Error estimates at 1*.