Manganese and iron (hydr)oxides are recognized to be important controls on the cycling of trace metals (Jenne, 1968). As such, the formation and dissolution processes of both (hydr)oxides are important in the study of environmental chemistry. Photoreductive dissolution for manganese and iron (hydr)oxides in the presence of organic substances has been well documented (Matsunaga et al., 1995; Waite and Morel, 1984a), however, far fewer investigations of photodissolution without organic substances have been undertaken, and mechanisms are less clear (Waite and Morel, 1984b). Photoreductive dissolution of amorphous MnO2 without organic substances was examined in this experiment.
Amorphous MnO2 was prepared in the laboratory(van der Berg and Kramer, 1979), and was characterized by X-ray diffraction, scanning electron microscopy, energy dispersive spectrometry and UV-Visible spectroscopy. Dissolution experiments at 27ƒC using 10-4 M MnO2 in quartz flasks were conducted on cloudless days in late July in Laramie, WY to determine the dependence of inorganic sunlight-induced dissolution on pH. Control experiments excluding sunlight were also run to monitor thermal dissolution. Photochemical dissolution rates were obtained by subtracting thermal dissolution rates from dissolution rates in the sunlight experiments.
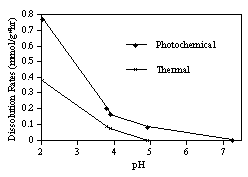
Dissolution of MnO2 occurred both thermally and photochemically; rates of both processes increased with decreasing pH (Fig. 1). At pH 2.1, the lowest pH in the experiment, the photochemical dissolution rate was 0.77 mmol/g×hour compared to the thermal dissolution rate of 0.38 mmol/g×hour. These results indicate that organic substances are not necessary for significant photodissolution of MnO2 to occur. Further experiments are planned to determine mechanisms of inorganic photoreductive dissolution.
Jenne, E.A., Adv. Chem. Ser. 73, 337-387 (1968).
Matsunaga, K., Ohyama, T., Kuma, K., Kudo, I. & Suzuki, Y., Wat. Res. 29, 757-759 (1995).
Waite, T.D. & Morel, F.M.M., J. Colloid Interface Sci. 102, 121-137 (1984a).
Waite, T.D. & Morel, F.M.M., Envir. Sci. Technol. 18, 860-868 (1984b).
van den Berg, C.M.G. & Kramer, J.R., Analyt. chim. Acta 106, 113-120 (1979).
Fig. 1: Thermal and photochemical dissolution rates of 10-4 M amorphous MnO2 at 27ƒC.