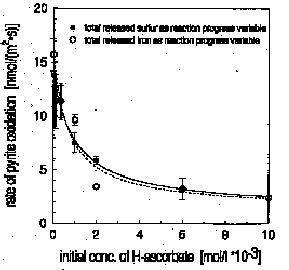
saturation in the presence of organic ligands and heavy metal ions. Reaction products such as sulfate, thiosulfate, total and soluble iron were analysed as a function of time. Rate constants of the surface controlled reaction were calculated from a rate law of pseudo-zero order. Non-reducing organic ligands with conditional complexation constants for Fe3+ between lgK=8 and lgK=14 at pH=7 accelerated pyrite oxidation. Fe3+-reducing ligands such as hydrogen ascorbate and tannic acid retarded or stopped pyrite oxidation depending on their intial concentration (see Fig. 1). The kinetics of hydrogen ascorbate oxidation in the presence of pyrite was examined in detail and is compared with the kinetics of the reaction of H-ascorbate with Fe(III) at pH=7 in the absence of pyrite. The retardation of pyrite oxidation by hydrogen ascorbate is explained as a reaction of hydrogen ascorbate with Fe(III) adsorbed at the surface of pyrite, i.e. the competitive reduction of the pyrite-oxidant Fe(III) by hydrogen ascorbate. The formation of a ternary surface complex between hydrogen ascorbate, Fe(III) and pyrite is discussed due to the fractional order both of pyrite oxidation in the presence of H-ascorbate and H-ascorbate oxidation in the presence of pyrite. Our results support a recently proposed mechanism of pyrite oxidation, according to which adsorbed iron is the direct oxidant and not oxygen.