Fibrous diamonds contain micro-inclusions characterized by volatile molecules and incompatible elements (Schrauder and Navon, 1994; Akagi and Masuda, 1988). IR study has identified CO2, carbonates in the diamonds and also disclosed that the inclusions keep high pressure corresponding to the environment of the growth (Navon, 1991), indicating the inclusions are firmly protected from volcanic events and weathering later on. The inclusions are considered to possess precious information on the environment of the upper mantle. The carbon isotopic study of the inclusions would shed light on the genetic relation between diamond, CO2 and carbonate. We have analyzed the carbon isotope of the three separately, compared them and discussed their relation.
CO2 in diamonds was extracted by crushing a sample under vacuum. It was collected and purified. The crushed diamond was removed into a reaction vessel and reacted with phosphoric acid under vacuum. CO2 released from carbonate was purified and the carbon isotope was measured. Chips or dust from the crushed diamond were reacted with CuO at 1000 šC under vacuum and the resultant CO2 gas was purified and the isotope ratio was measured. The blank of CO2 and carbonate extraction and purification procedure is smaller than one twentieth of sample.
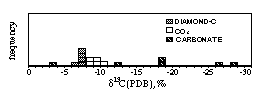
The carbon isotope ratios of diamond carbon and of CO2 are distributed in rather smaller range from -6 to -8” and from -8 to -10” in d13C (PDB) respectively, while that of carbonate scatters widely from -3 to -28” (Fig. 1). The accuracy of the data was examined from different aspects and no evidence against the widely scattering data was obtained. The carbon isotope of diamond carbon seems to correlate with CO2/(H2O+CO2) of the inclusion and that of CO2 correlates loosely with brightness of diamond specimens.
The some abundant and isotopic correlation between the diamond carbon and the CO2 imply at least "some" relation between them. The isotopic fractionation factor of carbon between CO2 and diamond, however, is positive (Polyakov and Kharlashina, 1995) and precludes the CO2 trapped in the cubic diamonds as the source of the diamond. Most provably the CO2 was trapped during aggregation of micro diamond crystals which had gravitated from a different place, and the carbon of diamond and CO2 is gradually exchanged changing isotopic composition of each carbon.
The present results evoke many carbon sources in the upper mantle. Among the heterogeneous carbon sources, the carbon for CO2 and diamond carbon are relatively homogeneous, which is a good contrast of the heterogeneity of carbonate carbon. Carbonate would be produced in the upper mantle utilizing any carbon including biogenic carbon. Carbonate thus generated would lose mobility and generate the carbon heterogeneity. CO2, on the other hand, could be mixed well in the mantle. The CO2 and H2O are dissolved in kimberite magma and would be the source of the carbonate xenolith of kimberlite.
Akagi, T. & Masuda, A., Nature 336, 665-667 (1988).
Navon, O., Nature 353, 746-748 (1991).
Polyakov, V.B. & Kharlashina N.N., Geochim. Cosmochim. Acta 59, 2561-2572 (1995).
Schrauder, M. & Navon, O., Geochim. Cosmochim. Acta 58, 761-771 (1994).
A review of published data for the volatiles N, CO2, H2O, F, Cl, Br, I and S shows that their concentrations in mantle-derived magmas vary with tectonic environment, geographical location, and the concentrations of other (non-volatile) components. Combined with data for the crust, hydrosphere and atmosphere, this information can be used to calculate volatile concentrations in the earth's primary (undifferentiated) mantle. The calculated primary mantle concentrations include (in ppm) 1.5 N; 335 CO2 (where CO2 = total C); 673 H2O; 32 F; 20 Cl; 0.07 Br; 0.011 I; and 174 S. Although provisional, the estimates are useful as common normalizing factors that enable volatile elements to be considered within the same framework of conventions and ideas used for non-volatile elements. This is illustrated for primary mantle-normalized patterns for volatiles and (non-volatile) incompatible elements in mid-ocean ridge magmas (MORB), intraplate ocean island magmas (OIB), and the (combined) continental crust/hydrosphere (Fig. 1).
Fig. 1: Carbon isotope of fibrous diamonds and their inclusions.