Partition coefficients for Rb, Sr, Sm, Nd, U, Th, Pb, La, Ce, Ba, Nb, and Li between phlogopite and leucite-lamproitic melt were determined in experiments similar to those described by Foley (1989). The runs were performed in a piston-cylinder-apparatus at 15 kbar and 1040 - 1175°C. Phlogopite glass pairs were analyzed by electron-beam-microprobe (37 experiments) and by secondary ion mass spectrometry (3 experiments).
The resulting data set differs from the values published by Adam et al. (1993) and LaTourrette et al. (1995), in that the
D-values for Rb, Ba, Pb (2-4 times), Sr (5 times), REE, Th, and U (200 times) are significantly lower. DBa varies as a function of the Ti- and Al-contents of the phlogopites between 0.3 and 1.6, which is in good agreement with the observations of Guo and Green (1990). Similar behavior on a lower level is suggested for the elements Sr and Pb and maybe REE. This correlation may also explain the higher D-values for Ba, Sr, and Pb measured by the other groups mentioned above, because the phlogopites in their experiments contain large amounts of octahedral Al. Partition coefficients of Nb and Zr are similar to those from LaTourrette et al. (1995), whereas the values for Li are higher
(10 times). It seems that DLi decreases with increasing content of octahedral Al in phlogopite. In addition, DCs was found to be significantly lower than DRb (0.50 and 1.34, respectively).
The model of Blundy and Wood (1994) that relates element partitioning to crystal lattice-site parameters, has been
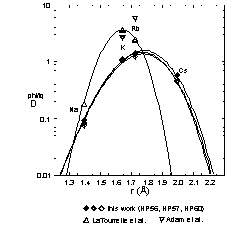
used to calculate the polyhedron Young's Moduli (E), the optimum ionic radius (r0) and the 'strain-compensated' partition coefficient (D0) for the X-site in phlogopite. The resulting values (using Na, K, Rb and Cs, see figure) are identical within error for all three experiments analyzed by SIMS: r0 = 1.773 ± 0.005 Å, E = 376 ± 6 kbar and D0 = 1.50 ± 0.10. The value obtained for E compares well with the Young's modulus (300 ± 45 kbar) that can be estimated (assuming that the coordination polyhedron behaves as a perfect solid) from the direct measurement of the interlayer polyhedron bulk modulus of phlogopite (Hazen and Finger, 1978). It is impossible to perform the same calculations with the D-values for the two 2+-cations Sr and Ba (Pb should not be used for such calculations because of its
non ideal behavior), but if r0 is assumed to be the same as for
1+- cations, then E and D0 can be calculated. In contrast to
the values for 1+ cations, there is a significant variation in D0 for 2+-cations, which varies between 1.82 (0.021 Al[IV]/22O in phlogopite) and 5.04 (0.180 Al[IV]/22O in phlogopite). There
is again no difference in E, the average value for the three experiments is 810 ± 21 kbar.
If this data set is compared with the corresponding values obtained by LaTourrette et al. (1995) there is very little agreement, since they found E's of 1000 and 1650 kbar for 1+ and
2+-cations, respectively, and a r0-value of 1.64 Å. If these values are used to calculated DCs, a partition coefficient at least two orders of magnitude lower than the measured value from this work is attained, contrasting with the occurrence of some tens of ppm Cs in natural phlogopite e.g. form the Finero peridotite.
A r0-value close to that from this work is suggested from the data of Adam et al. (1993) because their D-value for Rb is higher than that for K (5.8 and 2.7, respectively), although it is impossible to calculate it from the data given (only two
D-values for 1+-ions).
Adam, J., Green, T.H. & Sie, S.H., Chem. Geol. 109, 29-49 (1993).
Blundy, J.D. & Wood, B.J., Nature 372, 452-454 (1994).
Foley, S.F., In Kimberlites and related rocks (Ferguson, J., et al., eds.), Geol. Soc. Aust. Spec. Pub. 14, 616-631 (1989).
Guo, J. & Green, T.H., Lithos 24, 83-95 (1990).
Hazen, R.M. & Finger, L.W., Am Miner. 63, 293-296 (1978).
LaTourrette, T., Hervig, R.L. & Holloway, J.R., Earth Planet. Sci. Lett. 135, 13-30 (1995).