Submarine hydrothermal solutions are characterized by high acidity (pH=3-4), high Fe2+ concentration (up to 1-2 mM) and strong reducers (H2, CH4) abundance. During the mixing of the submarine hydrothermal solution with the sea water, the sharp pH increase and oxidizing of Fe2+ to Fe3+ form take place. As a result the solid iron (III) hydroxide is formed and it leads to coprecipitation of some trace elements (Feely et al., 1994; German et al., 1991).
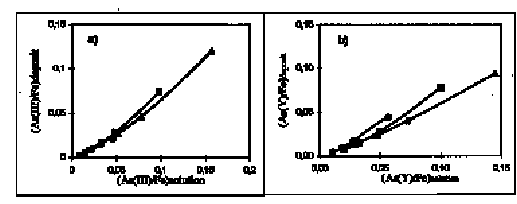
The experiments on modelling of As3+ and As5+ coprecipitation with iron (III) hydroxide under conditions of oxidation of dissolved Fe (II) in sea water were carried out. They allow to determine the relationship between element (i)/Fe ratio in solid phase and that of in initial solution:
(i/Fe)iron(III) hydroxide = k (i/Fe)initial solution
where i - As3+, As5+. The coefficient k is constant and is equal to 0.7, when i/Fe initial solution < 0.15. It proves the high efficiency of As3+and As5+ removal from the sea water with hydrothermal iron (III) hydroxide.
Assuming that As in metalloferrous deposits are connected with hydrothermal iron (III) hydroxide, the i/Fe ratio in
sediments was estimated on the base of experimental data. The results of calculations give the As/Fe ratio is 0.002-0.02. It is close to data, received from analysis of hydrothermal plume's suspended matter (0.002) and hydroxide fraction of metalliferrous deposits (0.001).
Feely, R.A., et. al., J.Geophys. Res. 99B, 4985-5006 (1994).
German, C.R., Campbell, A.C. & Edmond, J.M., Earth Planet. Sci. Lett. 107, 101-114 (1991).
Fig. 1: Dependence between atomic ratios (a) As(III)/Fe, and (b) As(Y)/Fe in iron(III) hydroxide deposit and that in initial seawater at pH = 7.7-7.8. Initial concentrations As(III), *M: 0.91 (Circles), 1.96 (Squares), 3.13 (Triangles). Initial concentrations As(Y), *M: 1.13 (Circles), 2.0 (Squares), 2.9 (Triangles).