aroostai@geol.uni-erlangen.de
The Upper Harz Mountains are characterized by predominantly westerly winds and annual precipitation rates ranging between 1550 and 1700 mm/yr. Altitudes vary from 400 m to 1140 m a.s.l. at the Brocken massif. The bedrocks consist of different varieties of Variscan granites and cover seven different hydrological catchment areas. In the upper part of this region swamps frequently occur. The area is neither directly influenced by local anthropogenic emissions nor by ore mineralizations.
During 1992 and 1993, 243 stream sediments and 318 water samples were taken at intervals of 0.5 and 1 km. Conductivity, pH and temperature measurements as well as wet sieving (< 63 mm) of the sediments were performed in the field. For monitoring and sampling of precipitations a bulk collector was used at an altitude of 700 m. To gain information about the background concentrations 49 bedrock samples were collected. The bulk depositions and stream waters were analyzed by ICP-MS and ion chromatography. Na and K were determined by flame photometry, Ca by ICP-OES. The bedrocks and sediments were analyzed by WDXRF for major and trace elements
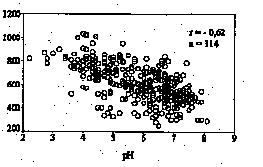
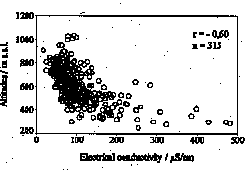
In the Harz Mountains, acid and heavy metal deposition is mainly caused by long-range atmospheric transport. The stream waters show extreme variations in concentrations. The median pH-value of the Brocken surface waters is 5.2, however, in the western part pH-values are about 1.5-2 units lower than in the eastern area. Low pH-values (2.8) were measured in the swamp areas. The pH-values and electrical conductivity negatively correlate with sample location altitudes (Fig. 1) and show seasonal and geographical
variations.
Most cation concentrations continuously increase with declining pH-values. These increases are dependent on the acid neutralisation capacity of soils and rocks. On the granites the soils exhibit no sufficient buffering capacity to neutralize the acid precipitation. Under these conditions the acidic waters dissolve silicates leaching Ca, Mg, Na, K, Ba and Rb. An insignificant amount of aluminum is released by weathering of silicates, whereas a considerable amount of aluminum and heavy metals are derived from high input of airborne particulates. This atmospheric deposition was found to be high for Al, Cd, Cu, Pb and Zn.
Compared to geogenic background samples (Brocken granite), the stream sediments are characterized by element contents which are lower by following factors: SiO2 (0.8), Al2O3 (0.7), Na2O (0.4), K2O (0.4), Ba (0.8), Rb (0.3). The concentrations of metals in the silt fraction of stream sediments, however, are higher than in the granite: As (5), Cd (13), Co (4), Cr (10), Cu (5), Ni (5), Pb (5), Zn (2) and Zr (5).
Stream waters show the following variations of heavy metal concentrations in mg/L for Cd (0.1-6.7), Co (0.03-4.6), Cr (0.01-4.3), Cu (0.01-18), Ni (0.01-33), Pb (0.08-41), Zn (1.3-643) and Al (5-1112). The concentrations of REE in stream waters are generally < 1 mg/L. Their distribution patterns are quite similar to those of the geogenic bedrocks. Swamp waters are depleted compared to the other surface waters in the area by the following factors: Na (0.8), Ca (0.3), Mg (0.4), Sr (0.4), Cl- (0.7), NO3- (0.6) and SO42 (0.6) and enriched with Al (1.4), As (1.7), Pb (4.3) and Zn (1.3).
The high concentrations of Al and heavy metals in stream waters of the Brocken area reflect the atmospheric input and chemical weathering of granite. The latter is accelerated by atmospheric acid deposition. Both the atmospheric acid supply and the low acid neutralisation capacity of the thin soil cover cause the strong acidification of the soil and aquatic environments.

Fig. 1: Relationship between pH resp. conductivity and the altitudes of water sample locations in the Upper Harz.