satr@esc.cam.ac.uk
The temperature-dependence of intracrystalline partitioning of metal cations over the M1 and M2 sites in olivine is a potential petrogenetic indicator. However, attempts to determine the temperature- and pressure-dependence of order/disorder over these sites, principally from the room temperature structures of annealed and quenched samples, give contradictory information about site
preferences of cation pairs. Recent studies show that considerable re-ordering occurs during quenching, thus high-temperature equilibrium states can only be determined in situ. A new model of the thermodynamics and kinetics of non-convergent ordering in (Fe0.5Mn0.5)2SiO4 olivine, determined by in situ neutron diffraction, allows the first estimate of the activation energy of Fe-Mn exchange (193±3 kJ/mole). It also reveals that the cooling rate of an olivine may be inferred from its room-temperature structure. The extension of this approach to rock forming olivines will provide an elegant geospeedometer for rocks which cool relatively rapidly.
In order to test the viability of olivine geospeedometry we chose first to focus on Fe-Mn intracrystalline exchange since Fe and Mn have a large contrast in incoherent scattering length for neutron diffraction. High-temperature neutron time-of-flight diffraction patterns of a synthetic (Fe0.5Mn0.5)2SiO4 olivine powder were collected at the Polaris diffractometer of the ISIS facility, Rutherford Appleton Laboratory. Full Rietveld structure refinements reveal significant changes in the M-site occupancies on changing temperature (Figure 1).
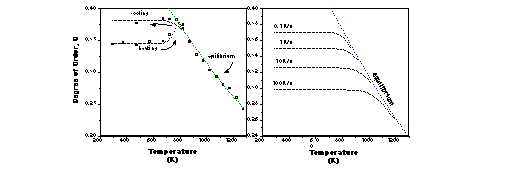
Upon heating, at around 400 °C the proportion of Mn in M2 begins to increase, until around 600 °C, above which it decreases continuously with increasing temperature. These higher-temperature data represent equilibrium states of order. On cooling, the degree of order saturates below equilibrium order at temperatures less than 500 °C.
Using time-dependent Ginzburg-Landau theory, we can simulate ordering paths expected at any cooling rate. Upon rapid cooling, Q , defined as (XMnM1-XMnM2)/(XMnM1+XMnM2) (i.e. Q = 1 for complete order and 0 for total disorder), falls below the equilibrium path at high-temperatures and a low value is frozen-in down to low temperatures. Slower cooling generates higher values of "quenched in" Q at room temperature. We refer to this as the saturation value of Q, Qsat, which increases with slower cooling rates. The M-site-occupancies measured at room temperature thus reflect the cooling rate of a quench. This reiterates the conclusion that published quench experiments on olivine do not describe the equilibrium high-temperature partitioning behaviour, but instead simply reflect particular cooling of individual experiments. Furthermore, this indicates that the room temperature structure of an olivine may be used as a geospeedometer for rapid cooling events, such as in volcanic rocks. The range of cooling rates is suggested by Figure 1, which shows the expected Q-T paths of (Fe0.5Mn0.5)2SiO4 olivine cooled at constant rates of 100 to 0.1 K.s-1. Adopting this method we find that the initial value of Q of our (Fe0.5Mn0.5)2SiO4 sample corresponds to a quench rate from its initial synthesis conditions of 1.5±0.2 K.s-1, which agrees with our estimate of the average quench rate of the sample from the synthesis temperature. We could equally easily determine Q-T paths following Newton's law of cooling, or other more elaborate time-temperature paths such as the step cooling path of our neutron diffraction experiment.
These results show that the cooling rate of an olivine may be determined from its room-temperature structure, specifically from the M1-M2 site occupancies. Nonconvergent ordering in olivines clearly provides a viable new method for high-speed geospeedometry. We are pursuing this approach and its application in planned and ongoing studies of Fe-Mg, Fe-Ni and Mg-Mn ordering in olivines.
Fig. 1: Q-T-t dependence in Fe:Mn olivine.