nives.ogrinc@ijs.si
Measurements of carbon isotope composition of porewaters in surficial sediments provides a way to study benthic carbon cycling, and also to gain knowledge of the factors which influence the carbon isotopic record in sediments. The chemical and isotopic analysis of pore water samples were performed in the central part of the Gulf of Trieste at the northern Adriatic (station F).
The sampling site is at the depth of 22 m and is composed of silty sand containing up to 80% carbonate. The sediments contain approximately 7 mg C/g dry weight as organic carbon. The composition of the sediment is practically uniform. The overlaying water is oxic throughout the year, except during late summer anoxic conditions were observed.
The isotopic composition of carbonate in Adriatic surface
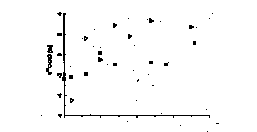
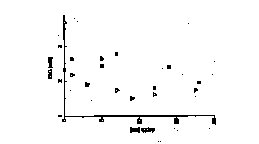
sediment in the Gulf of Trieste shows a variation of d18O values ranging between +26.27 and +28.82” (SMOW) and d13C values between -1.73 and +0.87” (PDB). This indicates carbon isotopic disequilibrium between atmospheric CO2 and sediment, so the resulting DIC should be isotopically lighter with d13C values ranging between -1.5 and -3.5” according to our model. Such an isotopically light DIC was in fact measured in the Gulf of Trieste. d13C-DIC values of pore water are shown in Figure 1. d13C values and the concentration of DIC in marine sediment reflect the contribution of organic derived carbon, mixing with DIC from the overlaying water column and CO2 from CaCO3 dissolution (McCorkle et al., 1985; McNichol et al., 1988; Bauer et al., 1995). The calculated saturation state indicates that carbonate minerals are dissolving through all sediment profiles during the whole year. In order to characterize the influence of diagenetic dissolution of CaCO3, analyses of the isotopic composition of CaCO3 were performed. d13C for bulk carbonate is essentially constant at +1.02” throughout the 20 cm sediment profile. To provide a basis for estimating the contribution of carbon from organic carbon oxidation, analysis of the isotopic composition of the bulk organic carbon were made. It was found that the d13C values of organic carbon (SOC) changed little with depth and time and the average d13C value is -21.9”. It is difficult to estimate which processes are responsible for the observed 13C-enrichment. In general, the oxidation of organic matter will increase the concentration of carbon in the pore water and decrease d13C-DIC. The dissolution of CaCO3 will also increase the concentration of DIC but has no effect on or slightly increases d13C-DIC. Exchange with bottom water will dilute the signal from oxidation by mixing in water with a low carbon concentration but high isotopic ratio. To understand the data, it is necessary to construct a model describing the processes which effect d13C-DIC in a coastal environment. An important factor in the sedimentary carbon cycle is dissolved organic carbon (DOC). It was observed that the concentration of DOC increases with depth. Furthermore, the low DOC/total alkalinity ratio, approximating a value of 1, which was calculated from the total alkalinity data, would suggest that DOC is produced almost entirely by sedimentation microbial processes (Lyons et al., 1982). The isotopic composition of DOC shows d13C values between -21 to -22”, corresponding to those of SOC.
With few exceptions, the concentrations of nutrients in pore water increase with depth, while those of PO43- show an irregular pattern with depth. In the late summer, the decrease in concentration of SO42- was observed and the concentrations of Fe2+ ions in pore water increased, which indicates anaerobic conditions.
The isotopic investigation of DIC was used as an indicator of processes affecting DIC in pore water. The d13C of DIC provides a powerful tracer of the oxidation of organic matter because the d13C of organic carbon is different from that of CaCO3 and bottom-water carbonate species. Further investigations are needed to explain the real source of 13C-enrichment of DIC.
Bauer, J.E., Reimers, C.E., Druffel, E.R.M. & Williams, P.M., Nature 373, 686-689 (1995).
Lyons, B.W., Gaudette H.E. & Gustafson, H.C., Org. Geochem. 3, 133-135 (1982).
McCorkle, D.C., Emerson, S.R., & Quay, P.D., Earth Planet. Sci. Lett. 74, 13-26 (1985).
McNichol, A.P., Lee, C. & Druffel, E.R., Geochim. Cosmochim. Acta 58, 2799-2809 (1988).

Fig. 1: The concentrations and *13C values of pore water DIC as a function of depth.