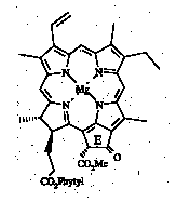
The major porphyrin in ancient sediments and oils is desoxophylloerythroaetioporphyrin (DPEP). The presence of an isocyclic ring suggests that DPEP is a diagenetic product of chlorophyll a (Fig. 1) (Treibs, 1936), as has been confirmed by the identification of sedimentary intermediates linking the precursor chlorophyll and product porphyrin (Keely et al., 1990). The secondmost abundant class of sedimentary porphyrin, aetioporphyrins, differ in that the isocyclic ring (ring E in structure 1) is absent. Accordingly, aetioporphyrins have been suggested to arise either from haeme pigments (Boreham et al., 1989; Callot et al., 1989) or from chlorophylls by way of cleavage of ring E (Corwin, 1960). Evidence from stable isotope analysis (12C/13C) suggests that both sources may be important (Boreham et al., 1989).
The presence of possible intermediates, so-called "altered chlorins" of the purpurin-18 (Fig. 2) or chlorin e6 (Fig. 3) type, in immature sediments was recognised by Baker and co-workers (see review in Baker and Louda, 1986) mainly from electronic absorption spectra of fractions and isolated components.
In order to evaluate the significance of chlorophylls as precursors to sedimentary aetioporphyrins we have examined lacustrine sediments from different settings. Analysis of the sediment extracts has been performed using high performance liquid chromatography (HPLC) with on-line diode array detection. Sedimentary components have been identified by comparison with standards, including a range of altered chlorins. Identifications have been confirmed using HPLC coupled with mass spectrometry. In two of the sites (Lake Baikal, Siberia and Loch Ness, Scotland) altered chlorins of the purpurin type are shown to occur. The presence of a range of purpurin derivatives indicates the operation of a chlorophyll transformation scheme involving cleavage of ring E. The purpurins are potential intermediates linking chlorophyll a and sedimentary aetioporphyrins and arise early in the transformation pathways of chlorophylls in aquatic environments.
Baker, E.W. & Louda, J.W., In Biological Markers in the Sedimentary Record (Johns, R.B., ed.) 125-225 (Elsevier, Amsterdam, 1986).
Boreham, C.J., Fookes, C.J.R., Popp, B.N. & Hayes, J.M., Geochim. Cosmochim. Acta, 53, 2451-2455 (1989).
Callot, H.J., Albrecht, P.A. Ocampo, R., Popp, B.N., Horowitz, M.R. & Hayes, J.M., Naturwiss. 76, 419-412 (1989).
Corwin, A.H., Proc. 5th World Petrol.Congr. Paper 10, 1-9 (1960).
Keely, B.J. Prowse, W. & Maxwell, J.R., Energy & Fuels 4, 628-634 (1990).
Treibs, A., Angew. Chem. 49, 682-686 (1936).
Fig. 1.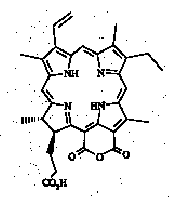
Fig. 2.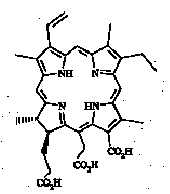
Fig. 3.