New experimental technique has been developed to measure the activity of water in H2O-CO2 mixtures over a wide range of temperatures and pressures. Water activities were monitored by the NiO+Ni-Pd sensor (Pownceby and O'Neill, 1994). Ag oxalate and water were weighed into Ag70Pd30 capsules containing two separate NiO+Ni-Pd alloys (in ZrO2 sleeves, Fig. 1) which bracket the expected composition after equilibration. If the two sensors converge on one composition then the run is considered a success, and the calculated fO2 based on that composition represents equilibrium.
Small capsules containing a range of H2O-CO2 mixes plus two NiO+Ni-Pd oxygen sensors were loaded into one large Ag capsule containing Ni+NiO+H2O and sealed. These capsules were held at P and T for two weeks in either cold seal pressure vessels, or piston cylinder apparatus. At the completion of the run the inner capsules were pierced and analyzed by weight loss, and confirmed via QMS analyses.
The method works by imposing a known fH2 via the Ni-NiO buffer in the outer capsule. Since: ![]()
![]()
and
![]()
the activity of H2O in the H2O-CO2 mix is simply determined by measuring fO2 with the Ni-Pd sensor.
The advantages of this technique over others used to the same end include: 1) a broad range of compositions can be equilibrated under the same P, T and fH2 conditions, 2) sensor reversals bracket water activities unequivocally, 3) the composition of the fluid can be accurately measured at the completion of the experiments, 4) experiments can be run over a broad range of pressures and temperatures.
Preliminary results using this technique indicate that aH2O in the system H2O-CO2 at 750°C and 2 Kb show complex deviations from ideality; with negative deviations from ideality at the water-rich end and positive deviations at the water-poor end.
Pownceby, M.I. & O'Neill, H.St.C., Contrib. Mineral. Petrol. 116, 327-339 (1994).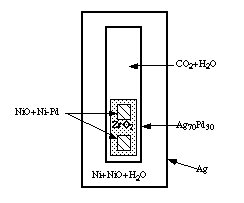
Fig. 1. Capsule configuration as described in the text. Note that numerous inner capsules are sealed into one large Ag capsule.