kadik @)glas.apc.org
The "intrinsic" fO2's recorded by the diamondiferous peridotite and eclogite xenoliths indicate that the redox state of the Archaean lithosphere is heterogeneous on scale of at least four log units mainly in the range between the WM and IW oxygen buffers. Highly reduced peridotites should be interpreted as a relict from earlier lower fO2 (Kadik, 1995).The fO2's recorded by the "fertile" and less modified spinel peridotite from Mongolia, Baikal and Then- Shan locations show that the redox state of the lithosphere beneath the territory of central Asia is heterogeneous on scale of two - three log mainly in the range between the WM and IW+1 oxygen buffers. This data provide evidence for the lower - fO2 regime of the carbon - bearing mantle beneath the territory of Baikal rift zone and Tien- Shan and the oxidation of diapirs ascending from the asthenosphere (Kadik, 1996). The "dry" xenoliths from Mongolia primarily reflect closed system behavior in the upper mantle the fO2. of which is buffered by ferric / ferrous redox equilibrium during cooling.
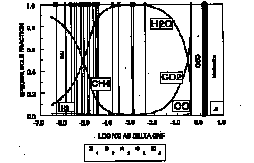
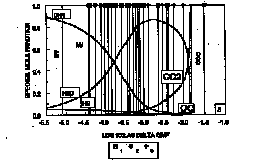
The trend of final fO2 data sets for the spinel peridotite xenoliths from Mongolia, Baikal and Tien-Shan cuts across the QMF and IW iron-bearing buffers and is not approximated by the vapor saturated graphite-CO2 surface or EMOG. Individual suites generally parallel the WM oxygen, but shifted to lower fO2 values. It may be suggested that this effect is result of closed-system subsolidus reequilibration between the phases during cooling and oxygen fugacity of the fertile upper mantle is buffered by ferric / ferrous redox equilibrium . Correlation between fO2 of minerals and the composition of the "dry" spinel lherzolites in terms of CPx abundance indicates decreasing of fO2 as extraction of the basaltic component increases. There are many possible explanations of the relationship between "fertility" and degree of oxidation. The most likely phase that could produce such an effect is graphite. It is expected (Kadik, 1995), that redox state of primary melt has been controlled by reactions: C (graphite) + 2Fe2O3 (melt) + O2- (melt) = CO32- (melt) + 4FeO (melt). The fO2's of the garnet lherzolites from Mongolia fall below the trend of the final data set for the spinel lherzolites It is assumed that changes in relative fO2 result from effect of phase transitions. The observed evolution of oxygen fugacities is closely linked to the distribution of volatile species in the mantle plums (Fig. 1A-B). H2O and CO2 would be dominant volatiles for more depleted and oxidized and CH4 -for more reduced and less modified part of peridotites. It is proposed that the upper mantle was originally more reduced and has become progressively more oxidized resulting perhaps largely from the preferred loss of hydrogen and carbon during melting. The oxygen budget of the upper mantle results from the opposing contributions of crustal recycling and carbon-bearing material transfer from the deep mantle.
This work was supported by International Sciences Foundation, grant MA8300.
Eggler, D. H.; Lorand, J..P; & Meyer, H. O.A., In Proceedings of the International-Kimberlite-Conference 5, 88-91 (1991).
Jaques, A.L , O'Neill, H.S., Smith C.B., Moon, J. & Chappell, B.W., Contrib. Mineral. Petrol. 104, 255-276 (1990).
Kadik, A.A., In Conference 0n Deep Earth and Planetary Volatiles. LPI Contribution No. 845, 106-114 (1995).
Kadik, A.A., Phys. Earth Planet. Inter. (1996, in press).
Kadik, A.A., Zharkova, Ye.V., Efimova, E.S. & Sobolev, N.V., Dokl. Akad. Nauk, Russia 328, 386-389 (1993a).
Kadik, A.A., Zharkova, Ye. V. & Spetsius, Z.V., Dokl. Acad. Nauk, Russia 337, 217-221 (1993b).
Kadik, A., A., Zharkova, He.V. & Kisililev, A.I., Dokl. Akad. Nauk, Russia 337, 100-103 (1994).
Kadik, A.A., Zharkova, Ye.V., Lutkov , V.S. & Tajibaev, G.T., Geokhimia, RAS N 8, 135-140 (1995).
Kadik, A.A., Zharkova Ye.V. & Kovalenko V.I., (1996, in press).

Fig. 1: (A, B) The composition of a separate C-O-H fluid in equilibrium with elemental carbon (graphite, diamond) plotted against of the fO2's (relative to the QFM ) recorded by diamond-bearing peridotite and diamond-bearing eclogite at 1100°C and 50 kbar (A) and "fertile" spinel peridotites (Baikal, Mongolia, Tien-Shan) at 1000°C and 15 kbar (B). A: (1) - diamond (Kadik et al., 1993a), (2) eclogite (Kadik et al., 1993b), Yakutia; (3) - peridotites (Kadik et al., 1993a), Yakutia; 3- diamond inclusions (Jaques et al., 1990), Australia; (4) - diamond inclusions (Eggler et al., 1991), Yakutia. B: 1- Mongolia (Kadik et al., 1996); 2-Tien-Shan (Kadik et al., 1995); 3- Baikal (Kadik et al., 1994).