The determination of growth and dissolution mechanisms of minerals in natural systems requires quantitative kinetic data in dependence of temperature, surface morphology or the chemical composition of solution (Bosbach and Rammensee, 1994; Jordan and Rammensee, 1996). The aim of our study is to present a method which quantitatively provides morphology and temperature dependent dissolution rates of single crystal faces by in-situ Scanning Force Microscopy. From these data the activation energy for dissolution can be determined and the course of rates vs. step density can be compared with theory. The method is also applicable to the investigation of crystal growth. In our study we illustrate the capability of SFM in resolving temperature and morphology dependent dissolution rates even in small temperature intervals by investigating dissolution of the (001) surface of brucite in acidic water (pH 2.7).
Experimentally, different solutions were supplied gravimetrically from beakers via a system of tubes, which enables an in-situ exchange of the solution applied, into the fluid cell of the SFM. Just in front of the microscope the solution
was heated up by immersing the supplying tube in water in a thermostated vessel. Heating up the fluid cell was done by introducing preheated saturated solution. After the desired temperatue in the fluid cell had been reached, the solution used for heating up was in-situ exchanged for the solution of interest. Temperature was controlled by a micro-NTC-resistor placed in the outdrop of the fluid cell, where the measured temperature had been calibrated for fluid flow and sample position. Using this arrangement temperature can be determined with an accuracy of at least ± 0.5 K.
Sequences of immediately successive images of the brucite (001) surface were taken during dissolution. In these sequences the differences of the areas of same terraces in successive images with same scan direction were calculated. The sum of these differences is the total dissolved terrace area per image area and time elapsed between recording the images. This sum is equal to the dissolution rate by converting the total dissolved terrace area into the number of moles. Dividing the dissolution rate by the mean total step length per image area of the corresponding images the mean step velocity can be obtained. The mean step velocity represents the velocity which would be required by a single step of total step length to dissolve the total dissolved terrace area. Finally both, dissolution rate and mean step velocity can be plotted against the mean total step length per area (or its reciprocal: the mean step distance) for different temperatures. Thus, the dependence of dissolution rates on surface morphology can be investigated. The error of these data depends on the image aquisition conditions and will be discussed in detail. Furthermore, especially in the case of possible interstep interaction these data are influenced by inhomogenious distributed steps in the scan field. Therefore in this case only image sequences with low inhomogenity of step density should be taken into account.
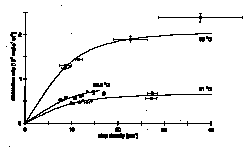
At step densities > 7 mm-1 the increase of dissolution rate of the (001) surface of brucite decreases with increasing step density. Therefore the dissolution rates approach a constant value. As shown in the figure, this non-linear behaviour is observable over the whole investigated range of temperature (21 - 35 ƒC) and gives evidence for interstep interaction. The estimated threshold of step distance for interstep interaction lies in the range of 80 - 100 nm. The activation energy for dissolution calculated from the dissolution rates at different temperatures is 60.2 ± 12 kJ mol-1. This value is noticeably higher than it will be if dissolution is transport controlled. A mean displacement of species adsorbed on the surface of a few tens of nm can be calculated by applying theory of surface diffusion (Chernov and Nishinaga, 1987) to the curves in the plot of dissolution rate vs. step density. Therefore we suggest that dissolution of the brucite (001) surface in acidic water (pH 2.7) seems to be rather surface controlled than transport controlled. An appreciable contribution of surface diffusion to the control of dissolution rate cannot be ruled out.
Bosbach, D. & Rammensee, W., Geochim. Cosmochim. Acta 58, 843-849 (1994).
Chernov, A.A. & Nishinaga, T., In Morphology of Crystals (ed. Sunagawa, I.) 207-267 (Terra Sci. Publ. Co., Tokyo, 1987).
Jordan, G. & Rammensee, W., Geochim. Cosmochim. Acta submitted (1996).
Fig. 1: Brucite (001) dissolution rates vs. step density at different temperatures.