Over the last decade, work on Platinum-Group Elements (PGE) has received a boost from new developments in analytical techniques. The distribution of PGE in the upper mantle can be provided by both the ultramafic xenoliths associated with kimberlites or alkaline volcanics and by the ultramafic orogenic massifs. The available data of PGE abundances in peridotites from the Betic-Rif range [Ronda (Spain), Beni Bousera (Morocco)] (Stockman, 1982; Gueddari et al., 1994) and ultramafic xenoliths from France (Massif Central and Languedoc) and Spain (Tallante) are still limited. The present work reports data on the PGE
abundances and provides the first detailed study of their distribution in these materials which have been affected by different degrees of partial melting. The object of this study is to compare PGE distribution in Ronda and Beni Bousera peridotites and xenoliths and to show evidence of the mantle heterogeneity. We discuss here the mechanisms of differentiation of PGE taking into account : 1) their geochemical affinities, 2) the abundances and distribution of PGE between the constituent phases of spinel peridotites,
3) oxygen fugacity conditions, 4) association of discrete grains of Platinum-Group Minerals (PGM) with sulphides.
PGE data concern 80 peridotites from orogenic massifs and in xenoliths. The PGE analysis was carried out by ICP-MS (Institut Dolomieu Laboratory) after wet-chemical extraction and enrichment by coprecipitation with selenium and tellurium (Amossé et al., 1986; Amossé, in prep.). The ICP-MS is calibrated by using a set of external standards (0, 2 and 20 ppb). The internal standards at a concentration of 100 ppb are 93Nb for Ru, Rh and Pd and 187Re for Ir and Pt.
In general, the PGE contents of the Ronda and Beni Bousera peridotites and of the xenoliths are about 10-3 times those of C1 chondrite of Naldrett and Duke (1980). The Ronda and Beni Bousera samples show a large overall amount of PGE (ÂPGE = 13.7-39.5 ppb) which is close to the abundance (ÂPGE) in xenoliths. However, the mantle-normalized PGE abundance patterns (Barnes et al., 1988) of Ronda and Beni Bousera peridotites are quite different than those of peridotite xenoliths.
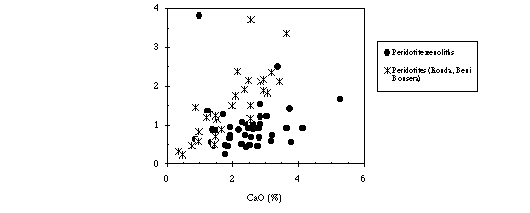
In the Ronda and Beni Bousera, the morphology of the patterns varies from unfractionated patterns to those with negative slopes. This variation is parallel with the residual character of the peridotites as shown by the decrease in the Pd/Ir mantle normalized ratios of the orogenic peridotites proportionally to their CaO contents (Fig. 1). This evolution illustrates the differential behaviour of PPGE (Pt, Pd) and Cu with respect to IPGE (Ir, Ru) and Ni during partial melting. As percentage of melting increases, the extraction of PPGE and Cu is favoured by the easy mobilization of these elements by sulphide liquid. By contrast, the IPGE are retained in the residues. The constituent minerals of spinel peridotites are characterized by very low PGE abundances (Table 1) indicating the Ir and Ru likely occur in intergranular phase. On the other hand, while in Beni Bousera peridotites Rh behaves like Pt and Pd and is extracted with sulphide liquid, in Ronda peridotites, this element tends to concentrate in depleted residual solid. The mechanism responsible of Rh contrasted behaviour (chalcophile at Beni Bousera and compatible at Ronda) do not involve different oxygen fugacities (fO2). The fO2 measurements (by using a solid electrolyte cell) and analyzed spinel (Table 1) prevent any suggestion of incorporation of Rh in the spinel as assumed by experimental data (Capobianco and Drake, 1990; Amossé and Allibert, 1992, 1993). In the light of these results, we suggest that the sulphur fugacity (fS2) in the Ronda is relatively lower than that in the Beni Bousera peridotites.
On the other hand, the PGE distribution of peridotite xenoliths is characterized by nearly flat mantle-normalized PGE abundance patterns. In opposition with orogenic peridotites, the Pd/Ir normalized-mantle ratios in xenoliths do not correlate with their CaO contents (Fig. 1). The geochemical evolution of PGE in the peridotites xenoliths is not controlled by partial melting processes. It may reflect either refertilization or retention of platinum and palladium minerals (observed in scanning electron microscope) in association with sulphides in a mantle more or less depleted. The flat PGE abundance patterns of the peridotite xenoliths do not represent necessarily a primitive upper mantle material.
The silicate minerals and spinel do not play a major role in PGE concentration. The absence of a "spinel effect" on the incorporation of Ir, Ru and Rh is in disagreement with some previous studies (e.g. Capobianco and Drake, 1990; Amossé and Allibert, 1992, 1993). The spinel could be represent the heritage of pre-existing phases (garnet and/or pyroxene?) which have no influence on the partitioning of these elements within the mantle during the retrograde evolution.
Differences in PGE behaviour between peridotites from orogenic ultramafic massifs (Ronda, Beni Bousera) or in xenoliths have an important consequence with regard to the mantle heterogeneity. This heterogeneity is herited by different physico-chemical and geodynamical evolutions.
Amossé, J., Fischer, W., Allibert, M. & Piboule, M., Analusis 14, 26-31 (1986).
Amossé, J. & Allibert, M. ,4th Symposium of Experimental Mineralogy, Petrology, and Geochemistry, Clermont-Ferrand, abstracts (1992).
Amossé, J. & Allibert, M., Geochim. Cosmochim. Acta 57, 2395-2398 (1993).
Barnes, S-J., Boyd, R., Korneliussen, A., Nilsson, L. P., Often, M., Pedersen, R. B. & Robin, B., In Geoplatinum 87 (Prichard, H. M., Potts, P. J., Bowles, J. F. W. & Cribb, S. J., eds.) 113-143 (London, Elsevier, 1988).
Capobianco, C. J. & Drake, M. J., Geochim. Cosmochim. Acta 54, 869-874 (1990).
Gueddari, K., Piboule, M. & Amossé, J., C. R. Acad. Sc. Paris 318, 79-86 (1994).
Naldrett, A. J. & Duke, J. M., Science 208, 1417-1424 (1980).
Stockman, H. W., Ph. D. Thesis, Massachussetts Institute of Technology, Cambridge (1982).
Fig. 1: Variation diagram of Pd/Ir vs. CaO in the peridotites from ultramafic massifs and in the xenoliths.Table 1: PGE abundances (in ppb) in constituent phases of spinel peridotites.
Ir Ru Rh Pt Pd
BS17 (Beni Bousera)
olivine 0.73 0.41 n.d. 0.07 n.d.
clinopyroxene n.d. n.d. n.d. 237 n.d.
spinel 4.1 n.d. n.d. 23.6 2.6
whole rock 3 7.6 0.79 7.2 1.5
JR34 (Ronda)
olivine n.d. n.d. n.d. n.d. n.d.
spinel 0.55 n.d. n.d. 5.1 0.35
whole rock 2.7 8.2 2.8 2.1 1.9
JR35 (Ronda)
olivine 0.76 n.d. n.d. 0.53 n.d.
orthopyroxene 6.1 1.4 1.5 92 3.3
clinopyroxene 3.5 n.d. 1.5 5.2 7.8
spinel 6.8 n.d. 33 27.3 32
whole rock 5.1 11.3 3.3 8.9 4.5
MB19 (xenolith)
olivine 0.52 n.d. 0.037 3.8 1.9
orthopyroxene 0.85 n.d. 0.07 1.1 1.5
clinopyroxene 1.3 0.26 0.3 4.6 3.4
spinel 2 n.d. n.d. 5.8 12.9
whole rock 3.9 4.9 0.86 6.2 3.6
n.d. : not detected