Ellis (1986) determined experimenally the Fe-Mg partitioning between garnet and silicate melts in the system SiO2-Al2O3-FeO-MgO-H2O. According to Ellis, the melt has a higher Mg/Mg+Fe-ratio than garnet (KD grt/m >1) at temperatures < 900 šC whereas KD grt/m is <1 above 900 šC. A significant compositional dependence of the partitioning coefficient was not observed. To check the reliability of the Ellis calibration in systems containig garnet in granitic melts we investigated the Fe-Mg partitioning in more complex systems containing 7 to 10 components including alkalis.
Hydrothermal experiments were perfomed at 5 kbar, 800-850 šC (fO2 imposed by Co-CoO-H2O-buffer) in order to syn-thesize garnet among other mafic crystalline phases in granitic melts. Starting materials were oxide mixtures in the SiO2-Al2O3-FeO-MgO-Na2O-K2O-H2O approaching the composition of metapelites. In series of runs we added TiO2, MnO, CaO and extra Al2O3. All starting compositions had an Mg/Mg+Fe ratio of 0.223.
Variation of the composition of the starting material results in considerable changes of the nature, the amount, and the composition of the crystallized phases (garnet, biotite, hercynite, orthopyroxene, orthoamphibole, cordierite, olivine, plagioclase). The compositional variability of the coexisting melt is relatively restricted. Major compositional changes were observed for the Or- and
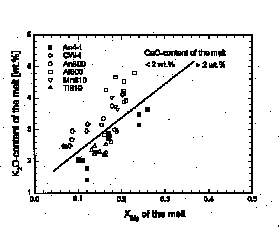
An-component and for the MgO-content of the melt. The MgO-content increases with increasing K2O- and CaO-content of the melt while the FeO-content of the melt is essentially constant. The Mg/Mg+Fe ratio is positively correlated to the K2O- and CaO-content of the melt (Fig. 1). The correlation affects the Fe-Mg parti-tioning between the mafic phases and the melt. Figure 2 shows the Fe-Mg partitioning coefficient for garnet and melt, KD grt/m ((FeO/MgO)grt/(FeO/MgO)melt) as a function of the Mg/Mg+Fe ratio of the melt for runs performed at 5 kbar and 800-810 šC. Increasing XMg in the melt results in an increase of KD grt/m. The results are explained with a compo-sitional dependence of the MgO-acitivty in the melt. Increasing K2O- and CaO-contents of the melt reduce the activity coefficient of the MgO-component. As a result the MgO-solubility in the melt increases at constant MgO-activity.
In various experimental investigations of the melting behaviour of metapelitic (Vielzeuf and Holloway, 1988; Le Breton and Thompson, 1988; Patino Douce and Johnston 1991, 1993), metapsammitic (Conrad et al., 1988), tonalitic (Skjerlie and Johnston, 1993) and amphibolitic rocks (Wolf and Wyllie, 1991, 1993) garnet appeares as hypersolidus phase. Figure 3 shows Fe-Mg partitionig coefficient for garnet and melt calculated from these investigations in the temperature range 850-875 šC at P=7-10 kbar in comparison with data of this study at 850 šC and 5 kbar. As in Fig. 2 there is a positive correlation between KD grt/m and XMg of the melt. The deviation of the two data points of Wolf and Wyllie (1991, 1993) from the general trend may be explained by the difference in the composition of their K-poor, amphibolitic starting material compared to the more acidic, K-rich starting material in the other investigations. Deviation of the garnet/melt Fe-Mg partitioning coefficient from the value determined by Ellis (1986) for the appropriate P-T conditions has been used as argument for chemical disequilibrium in some investigations (Le Breton and Thompson, 1988; Wolf and Wyllie, 1993). The results of our study demonstrate that this deviation is caused by the difference of the composition of the starting material (and hence the melt composition). There may be local equilibrium between garnet(-rim) and melt in the investigations cited above, although chemical equilibrium of the bulk system is usually not obtained as indicated by chemical zoning patterns in crystalline phases. Depending on the composition of the system KD grt/m = 1 can be obtained at temperatures different from the 900 šC as determined by Ellis (1986).
Conrad, W.K., Nicholls, I.A. & Wall, V.J., J. Petrol., 29, 765-803 (1988).
Ellis, D.J., Am. J. Sci., 286, 765-791 (1986).
Le Breton, N. & Thompson, A.B., Contrib. Mineral. Petrol., 99, 226-237 (1988).
Patino Douce, A.E. & Johnston, A.D., Contrib. Mineral. Petrol., 107, 202-218 (1991).
Patino Douce, A.E., Johnston, A.D. & Rice J.M., Am. Min., 78, 113-131 (1993).
Skjerlie, K.P. & Johnston, A.D., J. Petrol., 34, 785-815 (1993).
Vielzeuf, D. & Holloway, J.R., Contrib. Min. Petrol., 98, 257-276 (1988).
Wolf, M.B. & Wyllie, P.J., Mineral. and Petrol., 44, 151-179 (1991).
Wolf, M.B. & Wyllie, P.J., J. Geol., 101, 357-373 (1993).
Fig. 1: K2O-content vs. Mg/Mg+Fe ratio of melts in runs at 5 kbar and 800-810 šC. The diagonal line separates the symbols representing melts with CaO-contents > 2 wt.% from symbols representing melts with < 2 wt.% CaO. 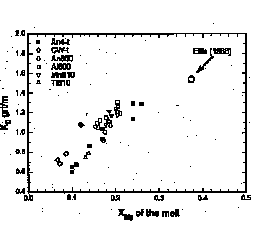
Fig. 2: Diagram showing Fe-Mg partitioning coefficients for garnet and melt (KD grt/m) vs. Mg/Mg+Fe-ratio of melts (XMg) in runs at 5 kbar and 800-810 šC. 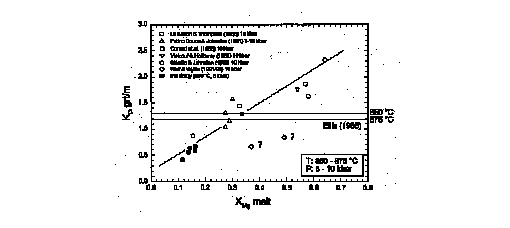
Fig. 3: Diagram showing Fe-Mg partitioning coefficients for garnet and melt (KD grt/m) vs. Mg/Mg+Fe-ratio of melts (Xmg). Data from the literature at P= 7-10 kbar and T= 850-875 šC are compared to own data obtained at 5 kbar and 850 šC. The two dotted horizontal lines for 850 and 875 šC indicate KD grt/m values calculated after Ellis (1986).