Biogeochemistry of Subsurface Environments: Investigation of Bacterial Effects on Iron Oxyhydroxide Coatings by Fluid Tapping Mode(TM) Atomic Force Microscopy
Meg C. Grantham School of Earth and Atmospheric Sciences, Georgia Institute of Technology, Atlanta, GA 30332, USA
dove@eas.gatech.edu
Patricia M. Dove School of Earth and Atmospheric Sciences, Georgia Institute of Technology, Atlanta, GA 30332, USA
Mineral surfaces in natural environments are often coated and cemented with iron (III) oxides. Since subsurface environments are often anaerobic as well as oligotrophic, biological contributions complicate the prediction of iron redox chemistry beyond the abilities of kinetic and thermodynamically-based models alone. Dissimilatory reduction of iron is often the preferred means for electron transfer in microorganisms in the absence of oxygen, but it also requires the availability of a suitable electron donor in the form of a metabolizable carbon source. Since there are often organic carbon source limitations in subsurface pore waters, the role of carbon availability on microbial metabolic-catalyzed dissolution reactions need to be considered.
Recent advances in Atomic Force Microscopy allow the in situ observation of living bacterial-mineral interactions by combining Tapping ModeTM and Fluid Cell technologies. This allows the determination of microbial effects on the dissolution of iron coatings and cements which exist in natural iron-rich quartz sands. Fluid Cell Tapping Mode Atomic Force Microscopy (Fluid TMAFM) was used in this study to investigate interactions of Shewanella putrefaciens with an iron-coated silica surface (as an analogue for quartz). S. putrefaciens are facultative anaerobic dissimilatory iron-reducing bacteria that are closely related to the genus Pseudomonas. These were seeded onto iron-coated and uncoated silica glass substrates in aqueous solutions of varying nutrient availabilities ranging from greatest to least in the order: Liquid Growth Media >> Synthetic Groundwater Solution >> Deionized H2O (CO2 = ambient). The seeded substrates were incubated under aerobic conditions for 1-4 days. Seeded and control samples were
examined using both Air and Fluid TMAFM. Images obtained using Fluid TMAFM were comparable to images obtained by Air TMAFM. Observations of live bacteria-surface interactions found that bacteria seeded in nutrient-depleted solutions adhered to iron-coated substrates more strongly than bacteria seeded in liquid growth media. Also, they adhered more strongly to iron-coated than uncoated silica glass. Bacteria seeded in liquid growth media were mostly flagellated. This observation suggests that motility and adhesion are related. Under some conditions the bacteria created pits and eroded areas in the iron coating within
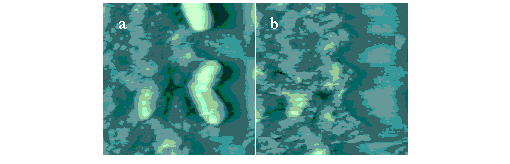
3 days. These features were revealed during sequential scans as bacteria were gradually removed from the surface. The adjacent figure shows Fluid TMAFM images of live S.
putrefaciens on iron-coated etched silica glass (image size = 8.75 mm). A bacterium is shown attached to the iron oxyhydroxide coating in Fig. 1a. An eroded area in the iron coating is revealed in Fig. 1b where the bacterium was attached. We do not presently know if depressions form by biological reductive dissolution of iron, by a reduction in local pH through extracellular secretion of organic acids, by CO2 production, or some other mechanism. These findings suggest that adhesion, motility, and iron surface chemistry are interrelated in a subsurface environment where iron-reducing microorganisms are present. We are currently designing experiments which use iron-coated quartz to better represent the natural weathering environment in which iron-reducing bacteria live.