The large field of the liquid immiscibility has been established by us in the fluorine-bearing granite system at 1 kbar and
in the range 650-1000oC (Gramenitskiy and Shchekina, 1993). It is connected with the limited fluorine solubility and the
separation of the alumino-fluorine ("fluid") melt from the aluminosilicate one. Na and Li are concentrated in the fluid melt and K - in the aluminosilicate one (Gramenitskiy et al., 1993). It has been shown for usual granite melt that Li and Sc were concentrated in the fluid melt, the most of other elements - in the aluminosilicate melt, but with different partitioning coefficients varying from very large to close to one. W, Sn, Zn, Pb partition coefficients (aluminosilicate melt/ fluid one) decrease in the
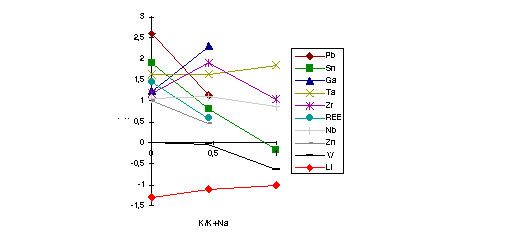
K-rich part of the system (up to 0.23; 0.69; 2.9; 13.8 correspondingly), and for Li, Ga - in the Na-rich one (see fig). The
introduction of Li into the system changes abruptly the partitioning coefficients of many elements: W, REE, Y, Zr, Hf, Pb, Zn are taking coefficients in favour of the fluid melt. Nb, Ta though prefer not to change their coefficients. Some changes also take place in going to nepheline normative area and to plumasite area of the system. The experimental data provide that the most efficient ore components concentration mechanism at the magmatic stage can be a separation of fluid melts. The data explain also some changes of geochemical indicators: Zr/Hf, Ta/Nb, REE spectra.
The work is supported by Russian Foundation of the Fundamental Researches.
Gramenitskiy, E. N. & Shchekina, T. I., Geochimija N 6, 821-840 (1993).
Gramenitskiy, E. N., Shchekina, T. I, Berman, I.B. & Popenko, D.P., Doklady Akademii Nauk (Russia) 331(1), 87-90 (1993).
Fig. 1: The alkaly ratio dependence of partitioning coefficients.