Amphiboles of the tremolite-richterite-K-richterite ternary occur in a variety of geological environments, such as contact metamorphic cherts, hyperpotasic subvolcanic and volcanic rocks, blueschist facies manganese deposits, and upper mantle xenoliths. Their genesis is believed to be related to metasomatic processes, and richteritic amphiboles are also regarded as potentially important reservoirs for trace elements and fluids within the earth's mantle. It was observed that the NaCl/KCl ratios in fluid inclusions of the Bufa del Diente contact-metamorphic aureole correspond to the composition of coexisting richterite solid solutions (Heinrich, 1994).
To test if the composition of richterite solid solutions can be used as a sensor for NaCl/KCl ratio in fluids the cation exchange equilibrium Na-richterite + KCl(aq) = K-richterite + NaCl(aq) has been investigated in hydrothermal experiments at 700 and 800 °C at 2 kbar. The exchangeable cations
Na + K were provided as excess 2n chloridic solution. The amphiboles grew in presence of the exchange fluid and therefore adjusted their stoechiometry in equilibrium with the fluid phase. The solid reaction products were more than 99 % amphibole (Na,K-richteritess) plus traces of diopside and quartz. Amphiboles produced by exchange syntheses were up to 1 mm long, and 40 mm thick. Detailed EMP- and HRTEM-observations showed that they are chemically homogeneous and structurally well ordered. The experimental results were reproducable and gave consistent phase relations in the reciprocal ternary system Na-richterite-K-richterite-NaCl-KCl. The Na-K distribution coefficients between fluid and amphiboles of the richterite-K-richterite join are close to unity at 700° C and 800° C at 2 kbar.
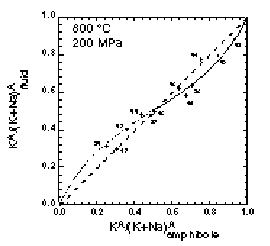
XKCl in fluid vs. XK-richterite plots at 700 and 800 °C show small but systematic deviations from the ideal mixing line (see Figure). The distribution coefficient KD of the exchange equilibrium for each experiment is not a function of XKCl in the fluid which indicates that the activity coefficients of NaCl and KCl can be assumed to be unity at the experimental conditions. KD, however, shows a linear correlation with XK-richterite. This implies that mixing on the A site in Richterite is not ideal. This observation is used to calculate a symmetric solution parameter for the A-position along
the richterite-K-richterite join. These experimental results actually show that the composition of richterite solid
solutions is a NaCl/KCl sensor for metamorphic fluids.
Heinrich W., Mineral. Petrol. 50, 259-270 (1994).